This Video Explains in detail how State of charge is calculated in a Victron BMV or smartshunt and or a Multiplus or Quattro Inverter charger, The Video will show you how to setup the Battery Monitor to suit your batteries condition and type of battery.
Note – this link (to the right) will take you to a Victron Energy page for BMV (battery Monitor) settings and what they do and mean CLICK HERE
Please be aware that The descriptions below relate to Lead Acid battery types.
Effect on capacity of rapid discharging
The capacity of a battery is dependent on the rate of discharge. The faster the rate of discharge, the less Ah capacity will be available. This is related to the diffusion process. In general the rated capacity is quoted for a discharge time of 20 hour Rate (discharge current I = C / 20).
For a 200 Ah battery this means that the rated capacity can be delivered at a discharge current of 200 Ah / 20 hours = 10 Ampères.
With a discharge current of 200 A the same battery becomes “flat” far sooner. For instance a 200 Ah gel battery then has an effective capacity of only 100 Ah and therefore becomes flat after 30 minutes.
The following tables give an impression of the capacity as a function of the discharge current.
The 2nd column of the first table gives the rated capacity as quoted by the manufacturer with the associated discharge time. Often this is 20 hour Rate, but it can also be 10 hour Rate or 5 hour Rate.
The tables show how capacity falls off steeply with increasing discharge current, and that AGM batteries (especially the spiral-cell battery) perform better than gel batteries under high discharge currents.

* With a discharge current of 1500 A (C / 1) the voltage of an A600 battery drops almost immediately to 1.65 V / cell (i.e. 9.9 V and 19.8 V for a 12 V respectively 24 V system).
Discharge current is often expressed as a proportion of the rated capacity. For example for a 200 Ah battery C / 5 means a discharge current of 40 A (= 200 Ah / 5).
Capacity and temperature
The effective capacity of a battery varies in reverse proportion to temperature:
– 10°C 10°C 15°C 20°C 25°C 30°C
80 % 92 % 95 % 100 % 103 % 105 %
1. The battery is discharged too deeply.
The deeper a battery is discharged, the faster it will age due to shedding, and once a certain limit is exceeded (approx. 80% depth of discharge) the aging process advances
disproportionately fast.
Additionally, if the battery is left discharged the plates will begin to sulphate.
A battery ages even when kept charged and doing nothing,
mainly due to oxidation of the positive plate grid.
The following table gives a rough idea of the number of charge/discharge cycles that batteries can withstand until the end of their service life, and how they could be destroyed by sulphation or due to plate corrosion.
Batteries are considered to have reached the end of their service life when the capacity they can hold has reduced to 80% of the rated capacity.

Although most batteries will recover from a full discharge, it is nevertheless very detrimental to their service life. Batteries should never be fully discharged, and certainly not left in discharged state. It should also be noted here that the voltage of a battery that is in use is not a good measure for its level of discharge. Battery voltage is affected too much by other factors such as discharge current and temperature.
Only once the battery is almost fully discharged (DoD 80% to 90%) will voltage drop rapidly. Recharging should have been started before this happens. Therefore a battery monitor is highly recommended to manage large, expensive battery banks effectively.
Charging too rapidly and not fully charging. Batteries can be quickly charged and will absorb a high charge current until the gassing voltage is reached. While charging with such high current might work well a few times, this will actually shorten the service life of most batteries substantially (the exception: spiral-cell and some other AGM batteries).
This is due to accelerated loss of cohesion of the active material, which results in shedding. Generally it is recommended to keep the charging current down to at most C / 5, in other words a fifth or 20 % of the rated capacity.
When a battery is charged with currents exceeding C / 5, its temperature can rise steeply. Temperature compensation of the charging voltage then becomes an absolute necessity
My own experience is that charging a 50 % discharged 12 V 100 Ah flooded battery at 33 A (C / 3) results in a temperature increase of 10 to 15°C. The maximum temperature is reached at the end of the bulk phase. Bigger batteries will become even hotter (because the amount of heat generated increases with volume and the dissipation of heat increases with the available surface) as well as batteries with a high internal resistance, or batteries which have been discharged more deeply.
An example:
Suppose a 50 foot sailing yacht has a 24 V service battery with a capacity of 800 Ah. The maximum charging current would then be C / 5 = 160 A. Then 320 Ah could be charged in 2 hours. If
simultaneously there is 15 A consumption, the charging equipment will have to deliver 175 A. During the remaining 22 hours of a 24-hour period an average of 320 Ah / 22 h = 14.5 A can be used, which means a discharge of only 320 / 800 = 40 %. This does not seem much, but unfortunately it is the maximum attainable when the generator period is limited to 2 hours. If used in this manner the cycling process will stabilise between a DoD of 20 % (beyond this point the charging voltage increases and the current accepted by the battery decreases) and a DoD of 20 % + 40 % = 60 %. Discharging more deeply and charging more rapidly would result in considerable loss of service life. In the example described above the battery is being used in partially charged state (between 20 % and 60 % DoD). Next to sulphation, there are two more reasons why the number of cycles in the partial state-of-charge mode should be limited:
1) Stratification of the electrolyte.
This problem is specific to batteries with liquid electrolyte:
As a rule of thumb, one should not extend partial state-of-charge operation beyond approx. 30 cycles, and much less in case of very deep discharges.
Cell unbalance.
Cells of a battery never are identical. Some cells do have a slightly lower capacity than others. Some cells will also have lower charge efficiency (see sect. 3.4.) than others. When a battery is cycled but not fully charged, these weaker cells will tend to lag further and further behind the better cells. To fully charge all cells, the battery has to be equalized (which means that the better cells will have to be overcharged,).
Unbalance will increase faster in case of very deep discharges or a very high charge rate. In order to prevent excessive cell unbalance, a battery should be fully recharged at least every 30 to 60 cycles.
Undercharging.
As discussed in section 2.2.4, sulphation will occur when a battery is left in fully discharged condition. Sulphating will also take place, although at a slower rate, when a battery is left partially discharged. It is therefore recommended to never leave a battery more than 50 % discharged and to recharge to the full 100 % regularly, for example every 30 days. Batteries, especially modern low antimony flooded batteries, often are undercharged because the
charge voltage is insufficient
Along with discharging too deeply, not fully charging is the major cause of premature aging of a battery.
Overcharging.
Charging too much is, in sequence, the 3rd main cause of service life reduction of a battery.
Overcharging results in excessive gassing and therefore loss of water. In wet batteries water loss
through excessive gassing can simply be replenished (yet the accelerated corrosion of the positive plates which takes place simultaneously is irreparable). However, sealed batteries which gas excessively cannot be replenished, and are therefore much more susceptible to overcharging. A frequent cause of excessive charging is the lack of temperature compensation or batteries being simultaneously charged using diode isolators
Temperature.
The temperature of a battery can vary greatly for various reasons:
– Rapid discharging and, to a much greater extent, rapid charging heats up a battery
– A battery’s location. In the engine room of a boat temperatures of 50°C or more can occur. In a vehicle the temperature can vary from – 20°C to + 50°C. A high average working temperature results in accelerated aging because the rate of the chemical decomposition process in the battery increases with temperature. A battery manufacturer generally specifies service life at 20°C ambient temperature. The service life of a battery halves for every 10°C of
rise in temperature.

Finally, temperature plays a big part in charging batteries. The gassing voltage and consequently the
optimum absorption and float voltages are inversely proportional to temperature.
This means that at a fixed charge voltage a cold battery will be insufficiently charged and a hot battery
will be overcharged.
Self-discharge
A battery at rest loses capacity as a consequence of self-discharge. The rate of self-discharge depends
on the type of battery and temperature.

When not in use, open lead-antimony batteries must be recharged after no more than 4 months, unless
the average ambient temperature is low.
Sealed batteries can be left without recharge for a period of 6 to 8 months.
When not in use for a long period of time, it is important to disconnect the battery from the electric
system, so that no accelerated discharging can take place as a result of current leaks elsewhere in the system.
Monitoring a battery’s state of charge.
‘The battery monitor’
The different ways of measuring a battery’s state of charge
Specific gravity (SG) of the electrolyte the electrolyte of a lead-acid battery consists of a mixture of water and
sulphuric acid. When fully charged, the active material in the negative plates is pure sponge lead; in the
positive plates it is lead oxide. The concentration of sulphuric acid in the electrolyte (and consequently
the SG) is then high.
During discharging the sulphuric acid from the electrolyte reacts with the active material in the positive
and negative plates forming lead sulphate and water. This reduces the sulphuric acid concentration and
consequently the SG of the electrolyte.
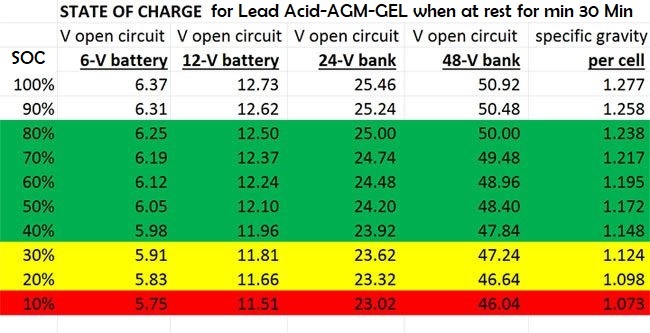
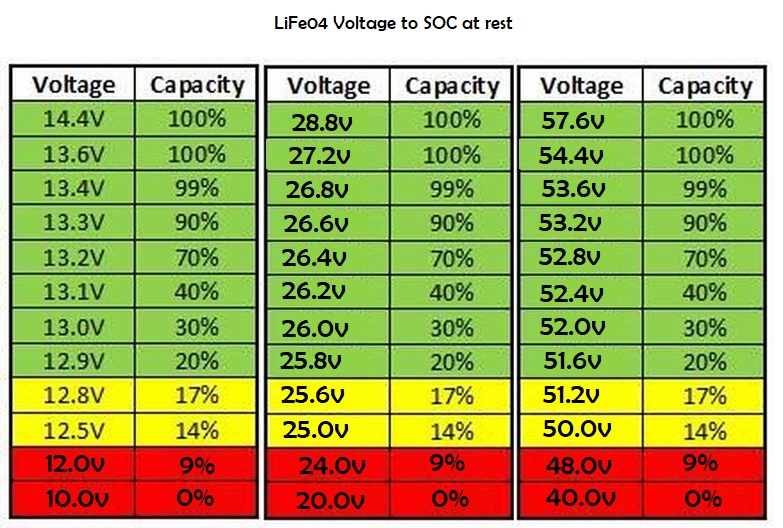
During discharging, the depth of discharge (DoD) of the battery can be tracked quite well by using a
hydrometer to monitor the SG of the electrolyte. The SG will decrease as shown in the following table:

During charging the reverse process takes place and sulphuric acid forms once again. Because
sulphuric acid is heavier than water, in batteries with liquid electrolyte (this does not apply for gel and
AGM batteries) it settles downwards, so that the acid concentration increases at the bottom of the
battery. However, above the plates the acid concentration in the liquid does not increase until the
gassing level is reached!
Some useful information about electrolyte:
– Stratification
Only once the gassing voltage (2.39 V per cell, or 14.34 V for a12 V battery at 20°C) is reached will the
electrolyte slowly become well mixed again by the gas bubbles.
The time needed depends on the construction of the battery and on the amount of gassing. The amount
of gassing in turn depends on the charge voltage, on the amount of antimony doping and age of the
battery.
Batteries with relatively high antimony doping (2.5 % or more) in general do gas sufficiently during the
absorption charge for the electrolyte to become homogeneous again.
Modern low antimony batteries (1.6 % or less antimony content) however gas so little that a normal
charge cycle is not sufficient. It then takes weeks of float charging (with very little gassing) before the
electrolyte is well mixed again. As a result flooded batteries, after having been fully charged, may
nevertheless show a low hydrometer reading!
Note: Vibration and motion in a boat or vehicle will in general adequately mix electrolyte.
– Temperature correction for hydrometer readings:
SG varies inversely with temperature. For every 14°C of temperature increase above 20°C, the
hydrometer reading will decrease with 0.01. So a reading of 1.27 at 34°C is equivalent to a reading of
1.28 at 20°C.
– Specific gravity variations per region:
The SG values as mentioned in the table above are typical for a moderate climate.
In hot climates SG is reduced as shown in the table below in order to diminish the effect of temperature
on service life of a battery
Fully charged SG, moderate climate: 1.265 – 1.285
Fully charged SG, sub tropical climate: 1.250 – 1.265
Fully charged SG, tropical climate: 1.235 – 1.250
Battery voltage too can be used as a rough indication of the battery’s state of charge (see preceding
Important: the battery should be left undisturbed for several hours (no charging or discharging) before a
valid voltage measurement is possible.
Amp-hour meter
This is the most practical and accurate way to monitor a battery’s state of charge. The product designed
for this is the battery monitor. The following sections look in more detail at the use of the battery
monitor.
The battery monitor is an amp-hour meter
The battery monitor’s main function is to follow and indicate the DoD of a battery, in particular to prevent
unexpected total discharge.
A battery monitor keeps track of the current flowing in and out of the battery. Integration of this current over
time (which if the current would be a fixed amount of amps, boils down to multiplying current and time) gives
the amount of amp-hours flowing in or out of the battery.
For example: a discharge current of 10 A for 2 hours means that the battery has been discharged by
10 x 2 = 20 Ah.
Energy efficiency of a battery
When a battery is charged or discharged losses occur. The total quantity of electric energy that the battery
takes up during charging is approx. 25 % greater than the energy given out during discharging, which means
an efficiency of 75 %. High charge and discharge rates will further reduce efficiency. The greatest loss occurs
because the voltage is higher during charging than during discharging, and this occurs in particular during
absorption. Batteries that do not gas much (low antimony batteries) and that have a low internal resistance are
the most efficient.
When a battery is used in the partial state-of-charge mode its energy efficiency will be quite high: approx. 89 %.
To calculate Ah charge or discharge of a battery, a battery monitor only makes use of current and time, so
compensation for the overall efficiency is not needed.
Charge efficiency of a battery
When a battery is charged, more Ah has to be “pumped” in the battery than can be retrieved during the next
discharge. This is called charge efficiency, or Ah or Coulomb efficiency (1 Ah = 3600 C).
The charge efficiency of a battery is almost 100 %, as long as no gas generation takes place. Gassing means that part of the charging current is not transformed into chemical energy that is stored in the plates, but used to decompose water into oxygen and hydrogen gas (this is also true for the “oxygen only” end of charge phase of
a sealed battery,
The “amp-hours” stored in the plates can be retrieved during the next discharge whereas the “amp-hours” used to decompose water are lost.
The extent of the losses, and therefore the charge efficiency depends on:
A. The type of battery: low gassing = high charge efficiency.
B. The way in which the battery is charged. If a battery is mainly used in partial state of charge and only charged up to 100 % now and again, the average charge efficiency will be
higher than if a battery is recharged to 100 % after each discharge.
C. Charge current and voltage. When charging with a high current and therefore also a high voltage and a
high temperature, gassing will start earlier and will be more intensive. This will reduce charge efficiency (and
also the overall energy efficiency).
In practice charge efficiency will range in between 80 % and 95 %. A battery monitor must take the charge
efficiency into account, otherwise its reading will tend to be too optimistic. If the charge efficiency has to be preset
manually it is advisable to initially choose a low value, for example 85 %, and adjust later to suit practice
and experience.
Effect on capacity of rapid discharging
As discussed in sect. 2.5.3. The capacity of a battery is dependent on the rate of discharge. The faster the rate
of discharge, the less Ah capacity will be available.
Back in 1897, a scientist named Peukert discovered that the relationship between the discharge current I and
the discharge time T (from fully charged to fully discharged) may be described approximately as follows:
Cp = In x T
where Cp is a constant (the Peukert capacity) and n is the Peukert exponent. The Peukert exponent is always
greater than 1. The greater n is, the poorer the battery performs under high rates of discharge.
Peukert’s exponent may be calculated as follows from measurements on a battery or using discharge tables or
graphs.
If we read (from a discharge table) or measure discharge time T1 and T2 for two different discharge currents (I1
and I2), then:
Cp = I
n
1 x T1 = I
n
2 x T2
and therefore:
n = log( T2 / T1) / log (I1 / I2)
As shown in the tables of section 2.5.3, increasing the discharge current from C / 20 to C / 1 (= increasing the
discharge current of a 200 Ah battery from 200 / 20 = 10 A to 200 / 1 = 200 A) can reduce effective capacity by
as much as 50 % for a mono block gel battery.
A battery monitor should therefore compensate capacity for the rate of discharge.
In practice this is quite complicated because the discharge rate of a house battery will vary over time.
Is capacity “lost” at high rates of discharge?
cites the example of a battery where the rated capacity under a 20-hour discharge was 200 Ah,
thus C20 = 200 Ah. The corresponding discharge current is:
I20 = C20 / 20 = 10 A
Under a discharge current of 200 A the battery was flat in 30 minutes. So although we started with a 200 Ah
battery, it was flat after discharging only 100 Ah.
This does not mean that, with a discharge current of 200 A, the 100 Ah capacity difference (C20 – C1 = 200 –
100 = 100 Ah) has “disappeared”. What happens is that the chemical process is
progressing too slowly, so that the voltage becomes unacceptably low. A battery discharged with 200 A and
“flat” in 30 minutes will therefore also be (nearly) fully charged again after recharging 100 Ah, while the same
battery which is discharged with I20 = 10 A and is flat in 20 hours will be nearly fully charged after recharging
200 Ah.
In fact a battery which has been discharged at a very high rate will recover over time and the remaining
capacity can be retrieved after the battery has been left at rest for several hours or a day.
23
Other Useful features of a battery monitor
In my opinion, apart from a voltmeter and an alarm function, very useful features are event counting and data
logging
Event counting
Event counting means that specific events; especially events that are potentially damaging or that on
the contrary are needed for battery maintenance are stored in a memory of the battery monitor.
Such events could be:
– over voltage
– under voltage
– number of charge-discharge cycles
– 100 % discharge
– 100 % recharge
Data logging
Data logging would mean that, in addition to specific events, at regular intervals the status of the battery
is stored in order to be able to reproduce a history of use at a later date.
